|
新冠病毒研發應用產品特輯 (一) 中和抗體篩選與效價評估 自 2019 年 12 月 COVID-19 武漢肺炎爆發以來,至今全球確診人數已突破 9,155 萬人 [1],各國政府與科研社群皆傾盡全力爭分奪秒地投入治療藥物與預防疫苗研發。本系列報導將透過近期發表文獻與您分享新藥開發技術領導品牌 Revvity 如何透過生物分子檢測技術與高通量檢測設備積極參與協助新冠病毒研究工作。本期將主要介紹中和抗體篩選與效價評估的應用產品與研究案例。 | |
以攜帶有 Luciferase 報導基因的 SARS-CoV-2 偽病毒,建立更安全便利的中和抗體檢測平台 |
|
|
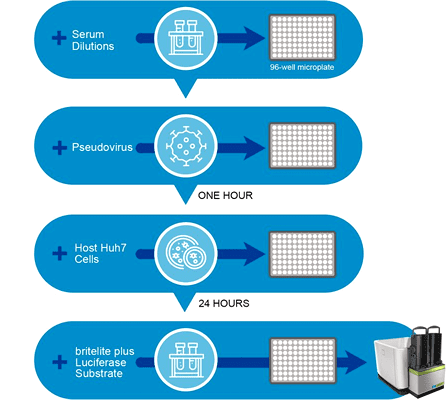
新型冠狀病毒 (SARS-CoV-2) 所具有的高度傳染性,使得科研人員必須在 P3 等級實驗室裡進行操作,此環境條件限制加上活病毒株取得不易等問題,都侷限拖緩了新冠病毒的研發腳步。為了讓科研人員能夠更安全便利地進行中和抗體藥物篩選和疫苗開發工作,中國食品藥品檢定研究院聶建輝等人建立了基於 SARS-CoV-2 偽病毒 (pseudovirus) 的中和抗體檢測平台,該平台於 P2 等級實驗室即可操作。他們首先利用基因轉殖技術將水泡性口炎病毒 (vesicular stomatitis virus, VSV) 進行改造,以螢火蟲冷光酵素基因 (firefly luciferase) 取代其原本的外套膜表面醣蛋白“G 蛋白”基因。接著將攜帶有 SARS-CoV-2 棘蛋白基因 (spike protein, S protein) 的 pcDNA3.1.S2 表現質體轉染至 293T 細胞中,再以基因改造病毒 (G*ΔG-VSV) 感染此細胞,即可生產出外套膜表面披覆有 SARS-CoV-2 棘蛋白的 SARS-CoV-2 偽病毒。 透過攜帶有 luciferase 報導基因的 SARS-CoV-2 偽病毒,作者建立了以細胞內 luciferase 表現量減少程度作為中和抗體活性指標的檢測平台,該平台採用 96 孔微量盤型式進行,每個 well 建議接種 5 x 10⁴ 的 Huh7 細胞與 650 TCID₅₀ 的 SARS-CoV-2 偽病毒,並以 britelite™ plus 冷光酵素受質以及 EnSight® 多功能微量盤檢測儀進行 luciferase 表現量分析與訊號偵測(圖 1)。作者以新冠肺炎痊癒者的恢復期血清與未染疫者血清驗證了此平台的效能與專一性,其結果數據可參見圖 2 [2]。
Fig. 1. Workflow for the pseudovirus neutralization assay for SARS-CoV-2. The EnSight® multimode plate reader offers best-in-class luminescence detection technology at high throughput rates (less than 1 min per 96-well plate) and can support biologics, vaccines, and small molecule research. The britelite™ plus homogeneous luciferase reporter gene assay system is designed to provide maximum signal intensity for assays requiring the utmost sensitivity. IMAGE © Revvity [3].
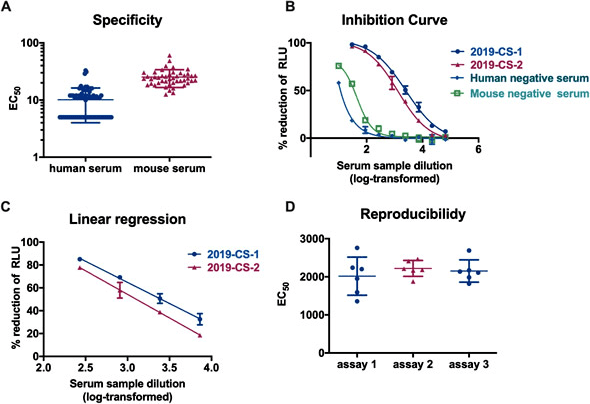
Fig. 2. Validation of the SARS-CoV-2 PBNA. (A) Specificity of the PBNA. A negative sample panel of seventy-four human and fortysix mouse serum samples were used to determine the specificity of this assay. (B) Inhibition curves for two SARS-CoV-2 convalescent patient serum samples and two negative serum samples derived from human and mouse. Typical four-parameter inhibition curves were observed for these two samples between log-transformed dilution and inhibition rate. The initial dilutions for positive and negative samples were 1:30 and 1:10 respectively, followed by 3-fold serial dilution. (C) Linear range for the PBNA. When the inhibition rate is in the range of 20%–80%, there is a linear relationship between the readout of the test sample and its dilution. (D) Reproducibility of the PBNA. A mixture of two SARS-CoV-2 coalescent patient serum samples was tested a total of 18 times on individual plates in three independent runs. IMAGE © Emerg Microbes Infect. 2020 Dec;9(1):680-686. Fig. 5 [2]. 中國北京大學未來基因診斷高精尖創新中心謝曉亮教授研究團隊使用單細胞定序技術從 60 位新冠肺炎痊癒者的恢復期血液中篩選出 149 種具有中和潛力的單株抗體,並沿用聶建輝等人建立的 SARS-CoV-2 偽病毒中和抗體檢測平台進一步驗證找出 7 種具有強力中和效果的單株抗體,其中表現最佳的抗體 BD-368-2 已經由動物實驗證實不僅具有治療效果,並且能對健康小鼠提供短期保護效果使其免於 SARS-CoV-2 感染。該研究成果已於 2020 年 5 月線上刊登於《Cell》期刊 [4]。 |
|
以免疫螢光染色與 EnSight® 多功能微量盤檢測儀,提升溶斑試驗與溶斑減少中和試驗 (PRNT) 的實驗效率與準確度 |
|
|
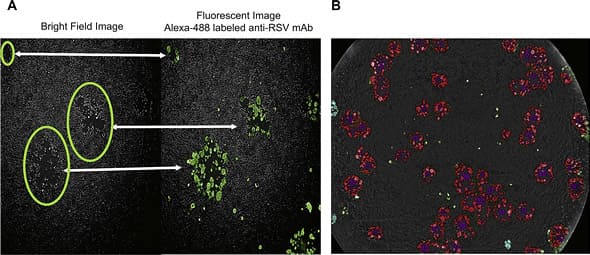
對於那些會造成感染細胞裂解 (lysis) 的病毒來說,溶斑試驗 (plaque assay) 是檢測病毒含量的黃金標準法。然而倚賴肉眼觀察與人工計數的溶斑檢測方式,往往耗時費力且容易產生人為誤差。默克藥廠 (Merck & Co., Inc.) Zhiyun Wen 等人利用免疫螢光染色技術結合 EnSight® 多功能微量盤檢測儀建立了溶斑試驗與溶斑減少中和試驗 (plaque reduction neutralization assay, PRNT) 的高通量檢測平台,該平台可大幅縮減檢測時間(以呼吸道融合病毒 (respiratory syncytial virus, RSV) 為例,可將原本耗時 5-7 天的檢測流程縮短至 3 天完成),同時透過 EnSight® 搭載的 Kaleido™ 影像分析軟體進行溶斑識別與自動化計數,提升實驗效率與準確度。 在進行溶斑計數時,Kaleido™ 軟體會先依據 EnSight® 擷取的光學影像圈選出溶斑位置,再透過病毒專一性抗體染色的螢光影像判別該溶斑是由病毒感染造成的專一性溶斑,或是其他因素(如未長滿的細胞或培養基內雜質)所導致的非專一性背景斑點(圖 3A)。圖 3B 則顯示 Kaleido™ 軟體能夠自動識別不同型態的溶斑,其中紅色代表溶裂型溶斑 (lytic plaques)、紫色為溶裂型溶斑的中心區域、青藍色則代表非溶裂型溶斑 (non-lytic plaques)。
Fig. 3. Generation of an automated counting algorithm. (A) Example of combined bright field image and fluorescence image contributing to the plaque counts. (B) Result from analysis of plaques by the algorithm. Plaque lytic centers are shown in purple. The corresponding fluorescence stained plaques are shown in red. Non-Lytic plaques are shown in cyan. The algorithm counts both lytic (red) and non-lytic (cyan) plaques. IMAGE © J Virol Methods. 2019 Jan;263:88-95. Fig. 4 [5]. 作者所建立的高通量溶斑試驗與溶斑減少中和試驗檢測平台,在實驗流程上主要有三個部分與傳統方式不同:(1) 由傳統的 24 孔微量盤型式轉換為 96 孔盤型式(以增加實驗通量)、(2) 移除病毒注入細胞前的細胞預培養步驟(可節省一天的培養時間)、(3) 將病毒與細胞混合液放入微量盤後,新增 10 分鐘的離心步驟(可增加實驗靈敏度),並在病毒與細胞混合培養後第三天即進行溶斑計數。 在檢測效能上,作者以平行實驗證實所建立的高通量檢測平台其溶斑試驗定量結果與傳統方式具有高度相關性;並以市售中和抗體藥物測試其溶斑減少中和試驗數據,也與過去發表文獻結果相符 [5]。雖然作者是以呼吸道融合病毒作為平台建立與條件測試的研究對象,然而相同的檢測平台建立模式將可同樣適用於 SARS-CoV-2,以加速其中和抗體藥物篩選與疫苗研發工作。 更多 SARS-CoV-2 研發相關產品資訊與應用實例,歡迎洽詢 Revvity 台灣代理 — 伯森生技。您可透過下方連結瀏覽更多相關資訊:
References
|
|

|
 |
伯森生物科技(股)公司 Blossom Biotechnologies, Inc.
網址 www.blossombio.com 客服 0800-059668
[ 📝 線上留言諮詢 ] [ ☎ 伯森業務專員聯絡資訊 ]